Abstract
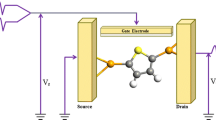
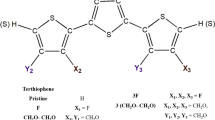
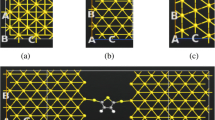
We report the charge transport phenomenon in polythiophene molecular device and the ways of controlling the nature of charge transport through the device. By using density functional theory (DFT) and non-equilibrium Green’s function (NEGF) formalisms, two ways of controlling the nature of charge transport have successfully been demonstrated by introducing conformational changes in the channel and applying external gate potential. Functional groups with negative mesomeric effect such as nitrous and carboxyl and positive mesomeric effect such as amino have been used as substituents as part of introducing conformational changes in the channel. The results indicate that the nature of charge transport in polythiophene molecular device can be changed from hole dominant to electron dominant and vice versa just by introducing minor conformational changes in the channel and by changing the polarity of external gate potential. Moreover, the negative differential resistance (NDR) behavior has been observed in amino-substituted thiophene device. These findings will be very useful in understanding the design of both p and n-type transistors out of same molecule for the next-generation molecular electronics.










Similar content being viewed by others
Data availability
All the related information is given in the manuscript and reference section.
References
Halbritter A, Sz C, Mihaly G, Jurdik E, Kolesnychenko OY, Shklyarevskii OI, Speller S, van Kempen H (2003) Transition from tunneling to direct contact in tungsten nanojunctions. Phys. Rev. B 68(3):035417. https://doi.org/10.1103/PhysRevB.68.035417
Nitzan A, Ratner MA (2003) Electron transport in molecular wire junctions. Science 300(5624):1384–1389. https://doi.org/10.1126/science.1081572
Reed MA, Zhou C, Muller CJ, Burgin TP, Tour JM (1997) Conductance of a molecular junction. Science 278(5336):252–254. https://doi.org/10.1126/science.278.5336.252
Ulman, Abraham. Formation and structure of self-assembled monolayers. Chem. Rev. 96, no. 4 (1996): 1533–1554. https://doi.org/10.1021/cr9502357
Du Y, Pan H, Wang S, Wu T, Feng YP, Pan J, Wee ATS (2012) Symmetrical negative differential resistance behavior of a resistive switching device. ACS Nano 6(3):2517–2523. https://doi.org/10.1021/nn204907t
Zheng X, Lu W, Abtew TA, Meunier V, Bernholc J (2010) Negative differential resistance in C60-based electronic devices. ACS Nano 4(12):7205–7210. https://doi.org/10.1021/nn101902r
Ratner MA, Aviram A (1974) Molecular rectifiers. Chem. Phys. Lett. 29(2):277–283. https://doi.org/10.1016/0009-2614(74)85031-1
Metzger RM (2003) Unimolecular electrical rectifiers. Chem. Rev. 103(9):3803–3834. https://doi.org/10.1021/cr020413d
Zeng J, Chen K-Q, He J, Zhang X-J, Sun CQ (2011) Edge hydrogenation-induced spin-filtering and rectifying behaviors in the graphene nanoribbon heterojunctions. J. Phys. Chem. C 115(50):25072–25076. https://doi.org/10.1021/jp208248v
Montiel F, Fomina L, Fomine S (2015) Charge transfer complexes of fullerene [60] with porphyrins as molecular rectifiers. A theoretical study. J. Mol. Model. 21(1):4
Mahmoud A, Lugli P (2012) Designing the rectification behavior of molecular diodes. J. Appl. Phys. 112(11):113720. https://doi.org/10.1063/1.4768924
Zeng, Jing, Ke-Qiu Chen, Jun He, Xiao-Jiao Zhang, and W. P. Hu. Rectifying and successive switch behaviors induced by weak intermolecular interaction. Org. Electron. 12, no. 10 (2011): 1606–1611. DOI: https://doi.org/10.1016/j.orgel.2011.06.010
Kiguchi, Manabu, Tatsuhiko Ohto, Shintaro Fujii, Kazunori Sugiyasu, Shigeto Nakajima, Masayuki Takeuchi, and Hisao Nakamura. Single molecular resistive switch obtained via sliding multiple anchoring points and varying effective wire length. Journal of the American Chemical Society 136, no. 20 (2014): 7327–7332. DOI: https://doi.org/10.1021/ja413104g
Larik, Fayaz Ali, Muhammad Faisal, Aamer Saeed, Qamar Abbas, Mehar Ali Kazi, Nadir Abbas, Akbar Ali Thebo, Dost Muhammad Khan, and Pervaiz Ali Channar. Thiophene-based molecular and polymeric semiconductors for organic field effect transistors and organic thin film transistors. Journal of Materials Science: Materials in Electronics 29, no. 21 (2018): 17975–18010
Kwon O, McKee ML (2000) Calculations of band gaps in polyaniline from theoretical studies of oligomers. J. Phys. Chem. B 104(8):1686–1694
Ma, Chang-Qi, Elena Mena-Osteritz, Tony Debaerdemaeker, Martijn M. Wienk, René AJ Janssen, and Peter Bäuerle. Functionalized 3D oligothiophene dendrons and dendrimers—novel macromolecules for organic electronics. Angewandte Chemie 119, no. 10 (2007): 1709–1713. DOI: https://doi.org/10.1002/ange.200602653
Rittmeyer SP, Groß A (2012) Structural and electronic properties of oligo-and polythiophenes modified by substituents. Beilstein journal of nanotechnology 3:909. https://doi.org/10.3762/bjnano.3.101
Maliakal A, Raghavachari K, Katz H, Chandross E, Siegrist T (2004) Photochemical stability of pentacene and a substituted pentacene in solution and in thin films. Chem. Mater. 16(24):4980–4986
Coppo P, Yeates SG (2005) Shining light on a pentacene derivative: the role of photoinduced cycloadditions. Adv. Mater. 17(24):3001–3005
Carraher Jr, Charles E. Seymour/Carraher’s polymer chemistry. Vol. 16. CRC press, 2003
Lee, Jeong-O., Günther Lientschnig, Frank Wiertz, Martin Struijk, Réne AJ Janssen, Richard Egberink, David N. Reinhoudt, Peter Hadley, and Cees Dekker. Absence of strong gate effects in electrical measurements on phenylene-based conjugated molecules. Nano Letters 3, no. 2 (2003): 113–117. DOI:https://doi.org/10.1021/nl025882
Matsushita R, Kaneko S, Nakazumi T, Kiguchi M (2011) Effect of metal-molecule contact on electron-vibration interaction in single hydrogen molecule junction. Phys. Rev. B 84(24):245412. https://doi.org/10.1103/PhysRevB.84.24541
Reddinger, Jerry L., and John R. Reynolds. Molecular engineering of π-conjugated polymers. In Radical Polymerisation Polyelectrolytes, pp. 57–122. Springer, Berlin, Heidelberg, 1999. DOI: https://doi.org/10.1007/3-540-70733-6_2
Hou, D., and J. H. Wei. The difficulty of gate control in molecular transistors. arXiv preprint arXiv:1109.5940 (2011). DOI: arxiv1109.5940
Xu Y, Fang C, Cui B, Ji G, Zhai Y, Liu D (2011) Gated electronic currents modulation and designs of logic gates with single molecular field effect transistors. Appl. Phys. Lett. 99(4):145. https://doi.org/10.1063/1.3615691
Mahmoud, Ahmed, and Paolo Lugli. Transport characterization of a gated molecular device with negative differential resistance. In Nanotechnology (IEEE-NANO), 2012 12th IEEE Conference on, pp. 1–5. IEEE, 2012. DOI: https://doi.org/10.1109/NANO.2012.6321941
Song H, Kim Y, Jang YH, Jeong H, Reed MA, Lee T (2009) Observation of molecular orbital gating. Nature 462(7276):1039. https://doi.org/10.1038/nature08639
Brandbyge, Mads, José-Luis Mozos, Pablo Ordejón, Jeremy Taylor, and Kurt Stokbro. Density-functional method for nonequilibrium electron transport. Physical Review B 65, no. 16 (2002): 165401. DOI: https://doi.org/10.1103/PhysRevB.65.165401
QuantumATK version Q-2016.4, Synopsys QuantumATK (https://www.synopsys.com/silicon/quantumatk.html)
Datta, Supriyo. Quantum transport: atom to transistor. Cambridge university press, 2005
Johannes G. Vos, Robert J. Forster, and Tia E. Keyes. Interfacial supramolecular assemblies. John Wiley & Sons (2003). DOI: https://doi.org/10.1002/0470861517
Liu, Zhen-Fei, and Jeffrey B. Neaton. Communication: energy-dependent resonance broadening in symmetric and asymmetric molecular junctions from an ab initio non-equilibrium Green’s function approach. (2014): 131104
Jiang, Zhuoling, Hao Wang, Yongfeng Wang, Stefano Sanvito, and Shimin Hou. Tailoring the polarity of charge carriers in graphene–porphine–graphene molecular junctions through linkage motifs. The Journal of Physical Chemistry C 121, no. 49 (2017): 27344–27350. DOI: https://doi.org/10.1021/acs.jpcc.7b09847
Kerber RC (2006) If it’s resonance, what is resonating? J. Chem. Educ. 83(2):223. https://doi.org/10.1021/ed083p223
Acknowledgments
The authors are thankful to Atal Bihari Vajpayee - Indian Institute of Information Technology and Management, Gwalior, for the infrastructural facilities provided to carrying out the present research work. One of us, Ankit Sirohi, is thankful to MHRD for a gate fellowship.
Author information
Authors and Affiliations
Contributions
All the authors have contributed equally in the reported work. Specifically the concept designing, analysis, and editing were done by Anurag Srivastava and Boddepalli SanthiBhushan, whereas the computation and initial writing were done by Ankit Sirohi.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
I, Anurag Srivastava, on behalf of all the authors, give consent for the publication of this work.
Competing interests
The authors declare no competing interests.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sirohi, A., SanthiBhushan, B. & Srivastava, A. Charge transport in polythiophene molecular device: DFT analysis. J Mol Model 27, 77 (2021). https://doi.org/10.1007/s00894-021-04680-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04680-w